STD X – PERIODIC TABLE, PERIODIC PROPERTY, VARIATION IN PROPERTY – NEWTON
About Course
In this section will learn the following chapters:
1.Introduction, periodicity, shells and orbits and valency
2.Periodic properties- part 1
3. Periodic Properties -Part 2, comparison of Alkali metals and Halogens
Last Updated:May 12, 2024
0 (0 Ratings)
Share Course
Page Link
Share on social media

Description
Periodic Table
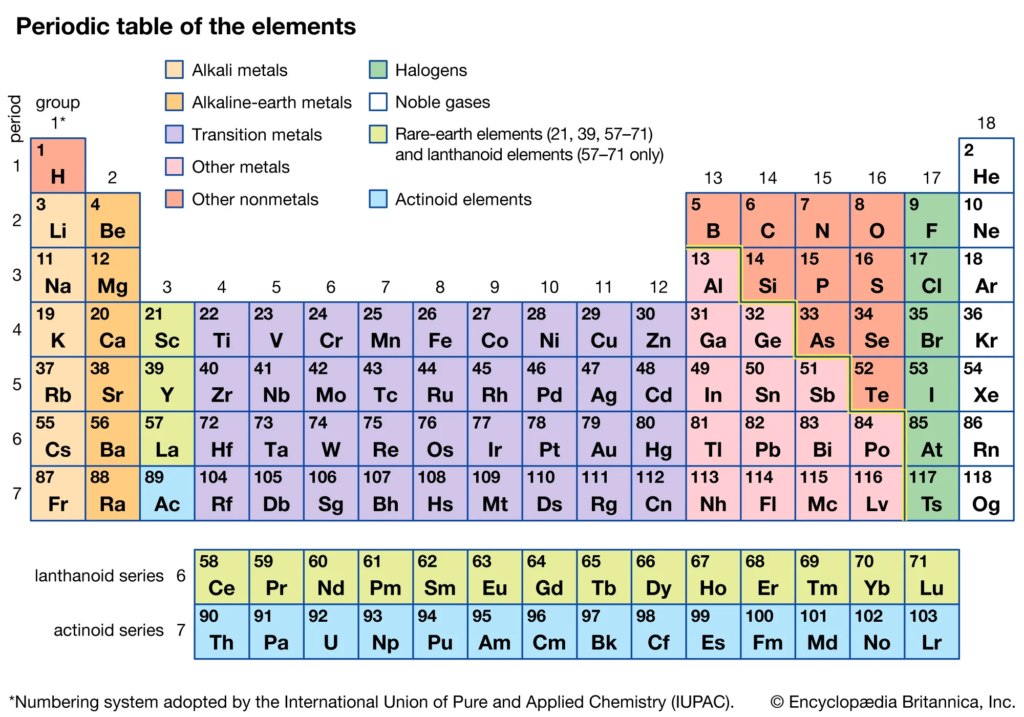

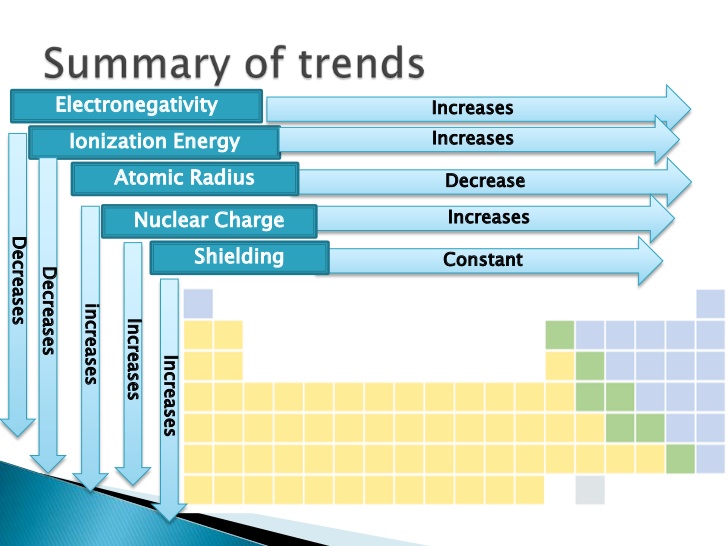
NOTE:
- Flourine is the most electronegative element
- Chlorine has the highest electron affinity
- Helium has the highest Ionization Potential
- Noble gases have the lowest Electron Affinity
Free
Free
Free access this course
-
LevelIntermediate
-
Total Enrolled3
-
Last UpdatedMay 12, 2024
Hi, Welcome back!
Material Includes
- 💻 Online recap of Lessons
- 📒 Worksheet questions and solution
- 📚 Testing and solution
- TEACHING AIDS:
- 🌺 Drawing periodic table with all elements and their electronic configuration
Course Duration:
0
Course level:Intermediate
Enrolled:3
About Course
In this section will learn the following chapters:
1.Introduction, periodicity, shells and orbits and valency
2.Periodic properties- part 1
3. Periodic Properties -Part 2, comparison of Alkali metals and Halogens
Course Curriculum
PERIODIC TABLE – PERIODICITY, METALLIC AND NON-METALLIC CHARACTER
-
[RHEA] [PHYSICAL CLASS] EARLY CLASSIFICATION AND PERIODICITY
17:24 -
[RHEA][PHYSICAL CLASS] VALENCY, NUCLEAR CHARGE AND ATOMIC SIZE
30:52 -
[RHEA] [PHYSICAL CLASS] METALLIC AND NON METALLIC PROPERTIES
28:08 -
[RHEA] [PHYSICAL CLASS] ELECTRON AFFINITY AND IONIZATION POTENTIAL
19:54 -
[RHEA] [PHYSICAL CLASS] TRENDS : ELECTRONEGATIVITY
14:30
PERIODIC TABLE – APRIL -2024
-
PERIODIC TABLE – PHYSICAL CLASS – DEPENDENCE OF CHEMICAL ACTIVITY ON ATOMIC SIZE AND NUCLEAR CHARGE
23:23 -
PERIODIC TABLE – PHYSICAL CLASS – INTRODUCTION TO THE FIRST 20 ELEMENTS AND PERIODS AND GROUPS
19:33 -
PERIODIC TABLE – PHYSICAL CLASS – IONIZATION POTENTIAL AND ELECTRON AFFINITY
23:23 -
PERIODIC TABLE – PHYSICAL CLASS – METALLIC AND NON METALLIC CHARACTER
23:10 -
PERIODIC TABLE- PHYSICAL CLASS – NUCLEAR CHARGE, ATOMIC SIZE AND VALENCY ACROSS GROUPS AND PERIODS
23:12 -
PERIODIC TABLE – PHYSICAL CLASS – REVISION OF CHARACTERS OF ELEMENTS 1 TO 20
23:23 -
PERIODIC TABLE – PHYSICAL CLASS – REVISION OF VALENCY, VALENCE ELECTRONS AND ATOMIC SIZE
18:15
Student Ratings & Reviews

No Review Yet

