STD VIII – PHYSICAL AND CHEMICAL CHANGES (Hybrid)
About Course
In this section will learn the following chapters:
1.PHYSICAL AND CHEMICAL CHANGES
2.CLASSIFICATION
Last Updated:September 5, 2023
0 (0 Ratings)
Share Course
Page Link
Share on social media
Description
Introduction
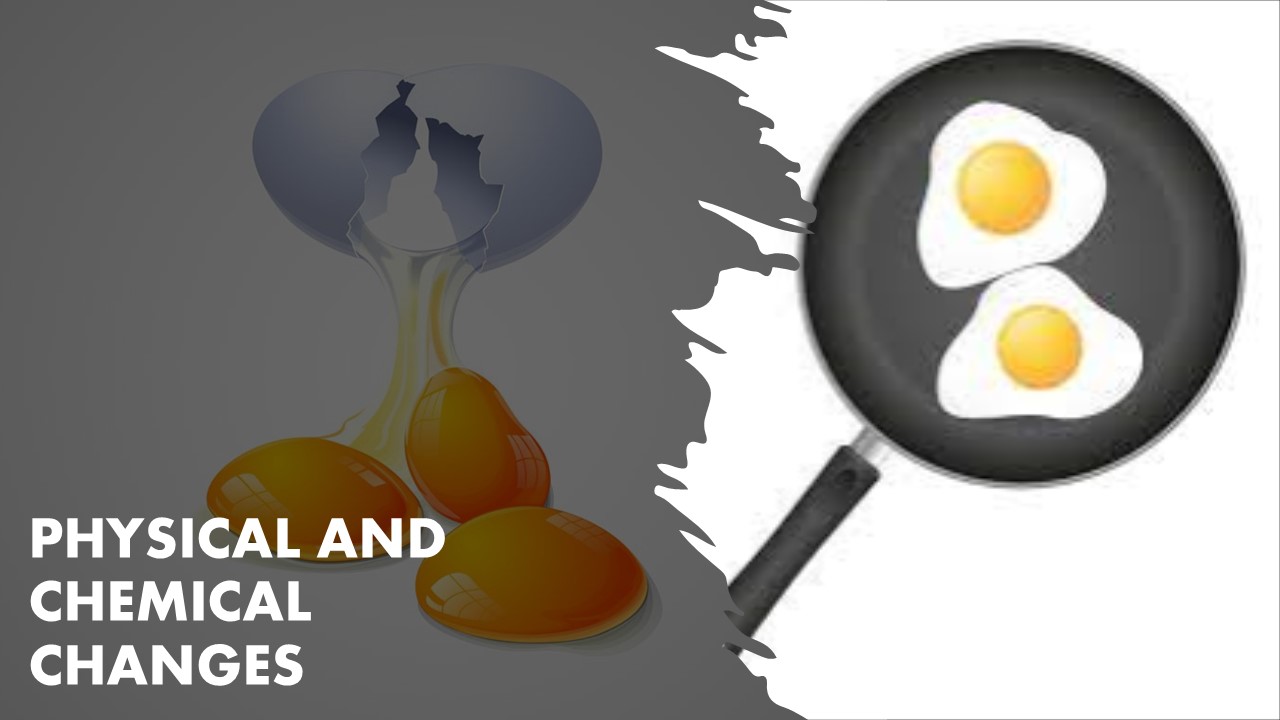
This theme will enable children to understand that there are different types of changes
in our surroundings which as slow/fast, reversible/irreversible, periodic/non-periodic and physical/chemical. In physical changes, no new substance is formed while in chemical change, a new substance with properties different from the element forming that substance is formed. Learning of these changes will also help in developing different scientific skills amongst them.
Physical Change
There is no addition or deduction of energy during the physical change, but the energy required for completion of change is released when the change is reversed.
Chemical Change
Energy like light, pressure, heat energy is required for chemical changes. Such energy is generally absorbed or released during the chemical change.
Examples of Physical changes:
Dissolving salt/sugar in water.
Sublimation of iodine.
Switching on an electric bulb.
Melting of solid/change in state of water.
Drying of wet clothes.
Examples of chemical changes
Burning of paper.
Burning of Magnesium in oxygen.
Cooking food.
Boiling/curdling milk.
Rusting of iron.
Free
Free
Free access this course
-
LevelIntermediate
-
Total Enrolled4
-
Last UpdatedSeptember 5, 2023
Hi, Welcome back!
Material Includes
- 🔥 Live Interactive classes with in-class doubt solving
- ⭐ Weekly Test and Quiz with instant tracking for progress
- ⚙️ Revision of the course after testing
- 👋 Fortnightly Parents and Tutor interactions
- 🌷 Expert monitoring of student's learning progress
- 👨👩👧👧 Daily communication over call, whatsapp and mail
- 💻3 hours on-demand video
- ✍4 downloadable resources
- ⌛Access for entire Academic Year
- 📱Access on mobile and Desktop
- 📋Assignments and review of the same
- 💡Tests and Correction by Board paper checkers
- 🏅Certificate of completion and Live tracking with Grade book
Course Duration:
0
Course level:Intermediate
Enrolled:4
About Course
In this section will learn the following chapters:
1.PHYSICAL AND CHEMICAL CHANGES
2.CLASSIFICATION
Course Curriculum
PHYSICAL AND CHEMICAL CHANGES
-
PHYSICAL AND CHEMICAL CHANGES – CORE CONCEPT – INTRODUCTION
01:48 -
PHYSICAL AND CHEMICAL CHANGES – CORE CONCEPT – SOME EXAMPLES OF PHYSICAL AND CHEMICAL CHANGES
09:21 -
PHYSICAL AND CHEMICAL CHANGE- SUPPORT MATERIAL – WHAT ARE CHEMICAL CHANGES
00:22 -
PHYSICAL AND CHEMICAL CHANGE – SUPPORT MATERIAL – ADDITIONAL TESTS
06:32
Student Ratings & Reviews

No Review Yet

